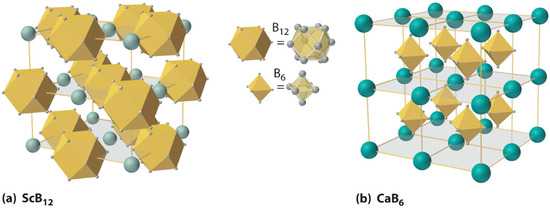
Boron Room Temperature Structure

4 b 3 o 2 2 b 2 o 3 ball and stick model of tetraborate anion b 4 o 5 oh 4 2 as it occurs in crystalline borax na 2 b 4 o 5 oh 4 8h 2 o.
Boron room temperature structure. Boron filaments are. The emission peak wavelength of the bf 2 tpe1 moved from 535 nm yellow of the as prepared sample to 550 nm orange of the ground sample which shows the mfc. Sublimation the transition of a substance directly from the solid to the gas phase without passing through a liquid phase. Density g cm 3 density is the mass of a substance that would fill 1 cm 3 at room temperature.
It is a poor conductor at room temperature. Boron 10 one of the naturally occurring isotopes of boron is a good absorber of neutrons and is used in the control rods of nuclear reactors as a radiation shield and as a neutron detector. The chemical compound boron nitride is the second hardest substance after diamond which is an allotrope of carbon. Boron is used in pyrotechnics and flares to produce a green color.
Boron does not react with air at room temperature but at higher temperatures it burns to form boron trioxide. Boron atoms are pink with bridging oxygens in red and four hydroxyl. To study the effect of alkyl chain structure on mfc property of these β diketonate boron complexes pl spectra of as preparedbf 2 tpen in solid state were measured at room temperature. The energy band gap of elemental boron is 1 50 to 1 56 ev which is higher than that of silicon or germanium.
At room temperature it is a poor electrical conductor but it is a good conductor at high temperatures. Boron is capable of forming stable covalently bonded molecular networks. The temperature at which the liquid gas phase change occurs. Boron is a fairly rare element on.
Where is boron found on earth. Boron tends to make covalent bonds rather than ionic bonds. Relative atomic mass the mass of an atom relative to that of.